A Complete Guide For Selecting The Best Clinical Trials Software Companies
Quick Summary: Selecting the best clinical trial software companies involves thorough research. Start by assessing each company’s reputation, considering client reviews and testimonials. Evaluate the software’s features, ensuring it aligns with your trial needs and regulatory requirements. Look for user-friendly interfaces and robust security measures, many other factors need to be considered. With this article, you will understand all the points that are necessary while selecting the best clinical trial management software companies. Keep reading!
Introduction
Medical research is advancing in all aspects. Thus, finding the right clinical trial software is essential for efficient and accurate data management and medical coding. Selecting the proper clinical trials sponsor data and software company is a critical decision that can significantly impact the efficiency and success of medical research. But that’s not as easy as it is a difficult task.
These points includes, Understanding your specific requirements. Different research projects demand distinct functionalities, so identifying unique needs will help narrow down the options. Consider factors such as therapeutic areas such as data management, regulatory compliance, financial management and user interface when assessing potential companies.
Additionally, Explore the reputation and experience of the clinical research operations software providers. Look for companies with a proven track record in delivering reliable solutions for clinical trials. Client testimonials and case studies can offer valuable insights into their performance and customer satisfaction. and many more…
and you will read all these necessary points in this article…
What Is Clinical Trials Software?

Let’s have a quick overview at what is CTMS?
Software for clinical trials is a specialized tool used in medical research to manage and streamline the process of testing new treatments or drugs on human subjects. It is also a type of clinical management solution or people also know it as a clinical trial management system. In addition, it helps researchers organize and collect data, track participant information, and ensure regulatory compliance for clinical studies. The software assists in protocol design, participant recruitment, data collection, and analysis, ultimately facilitating more efficient and accurate clinical trials which leads to better clinical care.
By centralizing information and automating various tasks of study management, clinical trial software contributes to the overall effectiveness and reliability of medical studies, leading to improved patient care and advancements in healthcare.
Why Selecting The Right Clinical Trial Software Company Important?

Here are several reasons why selecting the right Clinical Trial software Company Important:
Data Accuracy
Selecting the right clinical trial software company is crucial for ensuring data accuracy. Reliable software minimizes errors in data collection, entry, and analysis, providing precise information for researchers. This accuracy is fundamental for drawing valid conclusions and making informed decisions in the healthcare and pharmaceutical fields.
Compliance
A reputable clinical trial software company ensures compliance with regulatory standards for clinical applications. Adhering to guidelines and regulations is vital for the success and credibility of clinical trials. The right software automates compliance processes and manages clinical trials, reducing the risk of regulatory issues and ensuring that the study meets the necessary ethical and legal requirements.
Efficient Workflow
Choosing the correct software streamlines the workflow of clinical trials. Efficient processes enhance productivity, reduce delays, and optimize resource utilization. This leads to faster completion of trials, enabling quicker advancements in medical research and the availability of new treatments.
Security Measures
Security is important in clinical trials, where sensitive patient data is involved. The right software company implements robust security measures to safeguard patient confidentiality and protect against data breaches. This ensures the trust of the trial participants, and stakeholders in contract research organizations while maintaining the integrity of the research.
User-Friendly Interface
A user-friendly interface is essential for the effective use of clinical trial software. Intuitive design and ease of navigation contribute to user satisfaction and adoption. Researchers, clinicians, and other users can focus on their work without unnecessary complexities, leading to increased efficiency and successful clinical trial management system use.
Scalability
Choosing a software company that prioritizes scalability ensures that the system can adapt and grow alongside the evolving needs of your clinical trials. This flexibility allows for the seamless integration of additional features and managing an expanding volume of data and participants.
Technical Support
Adequate technical support is vital to address any issues promptly and maintain the smooth functioning of the software. A reliable support system minimizes disruptions, allowing researchers to focus on their work rather than troubleshoot technical problems.
Participant Recruitment and Retention
The software should facilitate efficient participant recruitment and retention through user-friendly interfaces and streamlined processes. This ensures a positive participant experience, contributing to the clinical trial management platform’s success and the generation of reliable data.
Real-time Monitoring
Real-time monitoring capabilities enable researchers to track, manage data capture, and analyze data as it is generated. This enhances the ability to promptly identify trends, anomalies, and critical information, leading to informed decision-making and timely adjustments to the trial protocols.
Collaboration and Communication
The software’s smooth collaboration and communication features foster effective teamwork among researchers, clinicians, and other stakeholders who conduct studies. This ensures that everyone involved in clinical research is well-informed and can collaborate seamlessly, promoting the overall success of the clinical trial.
Randomization and Blinding
Selecting the right clinical trial software company is important for efficient randomization and billing processes. Randomization is assigning participants to different treatment groups in a clinical trial. A software system ensures the random assignment of patients is accurate, reducing biases and enhancing the reliability of trial results.
Resource Optimization
The selection of an appropriate clinical research management and trial software company significantly impacts the optimization of resources. Clinical trials involve complex logistics, including personnel, equipment, and time. A well-designed software system efficiently allocates and manages these resources, enhancing trial productivity. This optimization leads to cost savings, faster trial completion, and improved data quality.
Criteria For Choosing The Top Clinical Trials Software Companies
By reading following points you will have a clear idea about how and what you need to keep in mind while you research sites selecting the best Clinical Trials Software Companies:
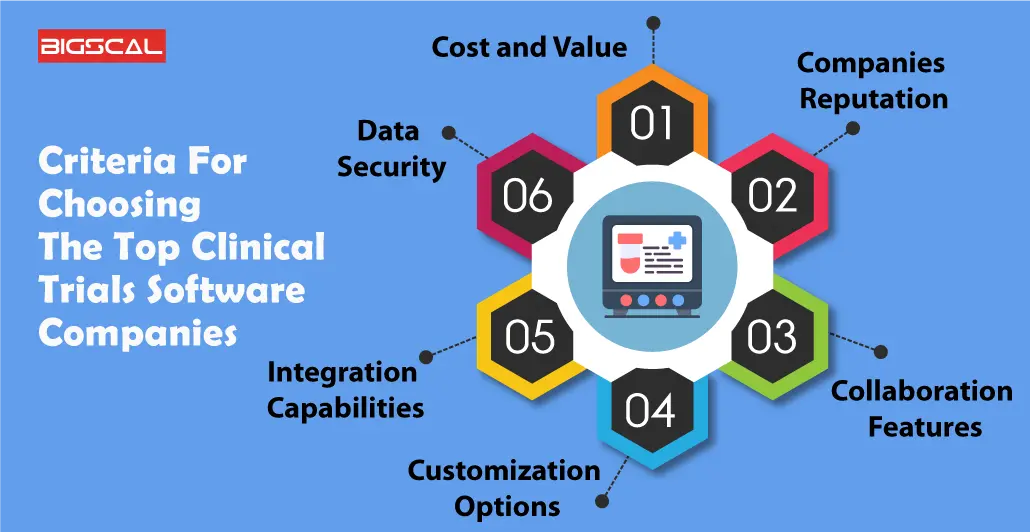
Cost and Value
Considering cost and value is crucial when selecting top clinical trial software companies. Evaluate pricing structures to ensure they align with your budget while providing essential features. Look for transparent pricing models, avoiding hidden fees. Assess the software’s overall value by weighing its key features up against the cost, ensuring it efficiently meets your trial management needs without unnecessary expenses.
Companies Reputation
A company’s reputation is a key factor. Research customer reviews, testimonials, and industry feedback to gauge the software provider’s reliability. A positive reputation indicates trustworthiness, quality, and customer satisfaction. Choose companies with a proven track record for delivering effective trial solutions and responsive customer support.
Collaboration Features
Effective collaboration is vital in clinical trials. Prioritize software with robust collaboration features, allowing seamless communication and data sharing among research teams. Look for tools supporting real-time collaboration, document sharing, electronic data capture, and secure communication channels. Enhanced collaboration features streamline trial management, fostering efficiency and ensuring all stakeholders are well-connected throughout the research process.
Customization Options
When selecting top trials software companies, customization options are crucial. This feature ensures that the software can be tailored to meet specific needs. Businesses vary, and a one-size-fits-all solution might not address unique requirements. Robust customization empowers users to adapt the software to their workflow, enhancing efficiency and user satisfaction.
Integration Capabilities
Integration capabilities are vital for seamless collaboration across different tools and platforms. A top trial software should easily integrate with existing systems, such as electronic health records or project management tools. This streamlines processes, reduces data silos, enables users, and promotes a cohesive ecosystem, allowing users to work efficiently without disruptions.
Data Security
Integration capabilities are vital for seamless collaboration across different tools and platforms. A top trial software should easily integrate with existing systems, such as electronic health records or project management tools. This streamlines processes, reduces data silos, and promotes a cohesive ecosystem, allowing users to work efficiently without disruptions.
List Of Best Clinical Trials Software Companies

BioClinica CTMS
BioClinica CTMS is a Clinical Trial Management System that optimizes various processes involved in clinical trials. It offers a user-friendly platform that enables efficient planning, tracking, and management of clinical trial activities. The software provides a centralized repository for essential trial information, allowing researchers and administrators to monitor progress, manage resources, and ensure compliance with regulatory requirements.
BioClinica CTMS facilitates collaboration among team members, enhances communication, and helps in making data-driven decisions. Its features include patient enrollment tracking, real-time data access, and customizable reporting tools, making it a valuable tool for research teams aiming to conduct clinical trials effectively and with precision.
IBM Clinical Development
IBM Clinical Development is a cloud-based platform that simplifies and accelerates the clinical trial process. It provides end-to-end solutions for planning, designing, and managing clinical trials efficiently. The platform enables seamless collaboration among research teams, allowing them to collect, analyze, and share data in real-time.
IBM Clinical Development emphasizes user-friendly interfaces and intuitive tools, making it accessible for both experienced researchers and those new to clinical trials. With features such as electronic data capture (EDC), randomization, and monitoring, the platform enhances data quality, reduces errors, and ensures regulatory compliance. IBM Clinical Development allows research organizations to conduct trials with precision, speed, and adherence to the highest industry standards.
EDGE
EDGE is a prominent clinical trials software company known for its solutions in research management. It offers innovative features that simplify the entire clinical trial process. With EDGE, researchers can efficiently manage protocols, track patient data, and ensure compliance with regulatory requirements. The software facilitates seamless collaboration among research teams, enhancing communication and data sharing.
Its intuitive design caters to users with varying levels of technical expertise, making it accessible for both professionals and those new to clinical research.
MasterControl CTMS Software
MasterControl CTMS Software is a leading solution for Clinical Trial Management Systems. This software excels in optimizing the planning, tracking, and management of clinical trials. It provides a centralized platform for organizing trial-related information, improving efficiency, and reducing errors. It’s user-friendly interface enables easy navigation and data entry, contributing to smoother trial workflows.
The software integrates with other systems, fostering connectivity promote collaboration across different stages of the trial process. With MasterControl CTMS Software, organizations can enhance their overall clinical trial management, ensuring compliance with regulatory standards while streamlining operations for more successful and efficient trials.
Dot Compliance
Dot Compliance systemizes regulatory compliance processes. The software assists in managing and organizing documentation essential for adherence to industry regulations. It simplifies tasks such as tracking protocols, billing compliance, managing study documents, and ensuring compliance with global standards.
Dot Compliance aims to enhance efficiency in clinical trials by providing a centralized hub for document management, reducing the risk of errors, and improving overall study quality. The platform’s intuitive interface makes it accessible for users across various roles within today’s clinical trials and research process, contributing to a more efficient and compliant research environment.
Clinical Conductor CTMS
Clinical Conductor CTMS is a comprehensive Clinical Trial Management System (CTMS) developed to optimize the planning and execution of clinical trials. This software is renowned for its ability to centralize and streamline the various aspects of trial management, including participant recruitment, site management, and financial tracking.
It facilitates communication and collaboration among research teams, allowing for real-time tracking of study progress and resource allocation. With user-friendly features, Clinical Conductor CTMS enables researchers to efficiently manage protocols, monitor site performance, and maintain compliance. The software’s capabilities contribute to improved operational efficiency, making it a valuable tool for organizations involved in clinical research.
Ripple
Ripple is a leading clinical trials software company providing innovative solutions for clinical trial management platforms and. With suitable interfaces, Ripple simplifies the complex process of clinical trial coordination. Its intuitive design enhances collaboration among research teams, facilitating efficient data collection and analysis.
Ripple offers features like patient recruitment tracking, data monitoring, and automated reporting, empowering researchers to focus on the scientific aspects of their work rather than administrative tasks. The software’s adaptability ensures seamless integration with various data sources, fostering a comprehensive approach to trial management. Researchers benefit from real-time insights, improving decision-making and overall trial efficiency.
RealTime CTMS
RealTime CTMS stands out as a robust Clinical Trial Management System (CTMS) solution. This software excels in centralizing trial data, providing a centralized hub for all trial-related information. It optimizes communication within the research team, enhancing collaboration and reducing errors.
RealTime CTMS offers features such as financial management, site performance tracking, and regulatory compliance tools. Its simple interface facilitates quick adoption by research personnel, contributing to smoother trial operations.
The software’s financial tools aid in budgeting and financial oversight, ensuring trials stay within allocated resources. RealTime CTMS is recognized for its comprehensive approach to trial management, catering to the diverse needs of research team and promoting transparency throughout the clinical trial process.
Ennov CTMS
Ennov CTMS is a leading clinical trial management system designed to streamline and optimize the complex processes involved in clinical trials. This software offers an interactive interface, facilitating efficient trial planning, tracking, and management.
Ennov CTMS enables researchers to oversee various aspects of the trial, from study planning to participant recruitment to data analysis. It promotes collaboration among team members by providing a centralized platform for real-time data sharing and communication—the system’s robust reporting tools aid in monitoring trial progress and compliance with regulatory requirements.
Ennov CTMS is more in demand due to its flexibility, capacity to accommodate diverse trial structures and adaptability to evolving research needs. With features like document management and electronic data capture, it contributes to the overall efficiency and success of clinical trials.
Florence eBinders
Florence eBinders is a comprehensive electronic trial master file (eTMF) system designed to simplify document management in clinical trials. This software excels in organizing and maintaining essential trial documents, ensuring compliance with regulatory standards.
Florence eBinders facilitates seamless collaboration among trial stakeholders, allowing for efficient document exchange and review. The platform’s user-friendly interface enhances accessibility and user adoption, contributing to smoother workflows. Its advanced tracking and reporting capabilities enable researchers to monitor document completeness and audit readiness.
Develop a software For Clinical Trial With Bigscal
If you are still confused and need customized clinical trial software then you must approach Bigscal technologies. Our software will streamline the complex process of managing clinical trials. We include modules for patient recruitment, data collection, monitoring, and reporting.
In addition, our developers make a system that will handle large-scale data, ensuring secure storage and quick retrieval. We Implement a user-friendly interface so that software will simplify data entry for researchers and healthcare professionals. Additionally, we also integrate the software with electronic health records and provide real-time analytics for informed decision-making.
This ensures that as the trial progresses, the software can accommodate growing data volumes without sacrificing performance. In conclusion, the proposed software harnesses Our power to enhance the efficiency, security, and scalability of clinical trial patient database management, ultimately contributing to advancements in medical research.
Conclusion
Choosing the right clinical trials software company is crucial for streamlined research processes, and that’s where this guide will help you. Evaluate potential providers based on their user-friendly interfaces, data security measures, and customization options. Prioritize companies with a proven track record in supporting diverse clinical trials. Additionally, consider scalability to accommodate future research needs.
FAQ
What software does clinical research use?
Clinical research utilizes various software, including Electronic Data Capture (EDC) systems like Medidata and REDCap for data collection, statistical analysis tools like SAS and R, and Clinical Trial Management Systems (CTMS) such as Oracle’s Siebel CTMS. Electronic Health Record (EHR) systems from pharmaceutical companies like Epic and Cerner are also essential for integrating patient data.
What is the system used in clinical trials?
Clinical trials rely on comprehensive systems like EDC platforms, which streamline data collection, management, and reporting. Popular EDC systems include Medidata, REDCap, and OpenClinica. Additionally, Clinical Trial Management Systems (CTMS) such as Oracle’s Siebel CTMS help oversee and coordinate various aspects of the trial, ensuring efficiency and compliance.
What is the difference between EDC and CTMS?
Electronic Data Capture (EDC) focuses on collecting and managing clinical trial data, ensuring accuracy and compliance. It handles data entry, validation, and storage. Clinical Trial Management Systems (CTMS) oversee the entire trial process, managing protocols, timelines, resources, and communication. While EDC is data-centric, CTMS is project-centric, ensuring effective trial management.
What is CRF software?
CRF (Case Report Form) software facilitates the design, creation, and management of electronic or paper-based forms used to collect patient data in clinical trials. It ensures standardized data collection, reducing errors and streamlining analysis. Common CRF software includes OpenClinica, Medidata Rave, and REDCap, enhancing data quality and trial efficiency.
What is trial management software?
Trial Management Software (TMS) is a comprehensive system designed to oversee and streamline various aspects of clinical trials. It facilitates protocol management, participant recruitment, site coordination, budgeting, and reporting. TMS enhances efficiency, compliance, and collaboration among stakeholders, ensuring the successful planning and execution of clinical trials.







