Most Demanding Clinical Data Management Software System For Upcoming Year
Quick Summary: To enhance clinical efficiency, we must do more medical device research. To handle all essential research data we need a Clinical Data Management Software to manage and organize all important data. So, get prepared about upcoming year’s best CDMS software for get more accuracy in clinical trials. Read on!
Introduction
As this year is about to end, we should be prepared for the upcoming year, 2024. To dominate the healthcare sector in the forthcoming year, you must know which clinical data management software is most in demand.
The recent research data explains the demand for clinical trial data management software solutions. The clinical trials are getting more complex. Thus, the need for exclusive clinical data management system software increases. The best data management in clinical trials simplifies data collection, integration, double data entry, and analysis.
You do not need to research which CDMS system is best for a clinical data manager for this year or next. We will discuss all the most demanding clinical data management tools for upcoming years.
Hang on and keep reading!
What Is CDMS- Clinical Data Management Software?

Clinical data management software is an essential tool for a clinic management system. It is also known as Clinical Research Management Software or the clinical data and laboratory information management system or CDM or trial data management software. In addition, it is used in healthcare and medical research to organize and handle clinical trials or patient studies data. CDMS is like a digital assistant for researchers and healthcare professionals. It helps collect, clean, and store large amounts of data efficiently, ensuring accuracy and compliance with regulatory standards.
This software streamlines the data entry process, reduces errors, and facilitates collaboration among parties. It also assists pharmaceutical companies in tracking the progress of clinical trials, managing patient information, and generating reports for analysis. Clinical data management software plays a crucial role in drug development and in maintaining the integrity and reliability of data throughout the lifecycle of a clinical study, contributing to the advancement of medical knowledge and the development of new treatments.
How Does Clinical Data Management Software (CDMS) Work?
After understand what is CDM, Let’s have a look at how CDMS work
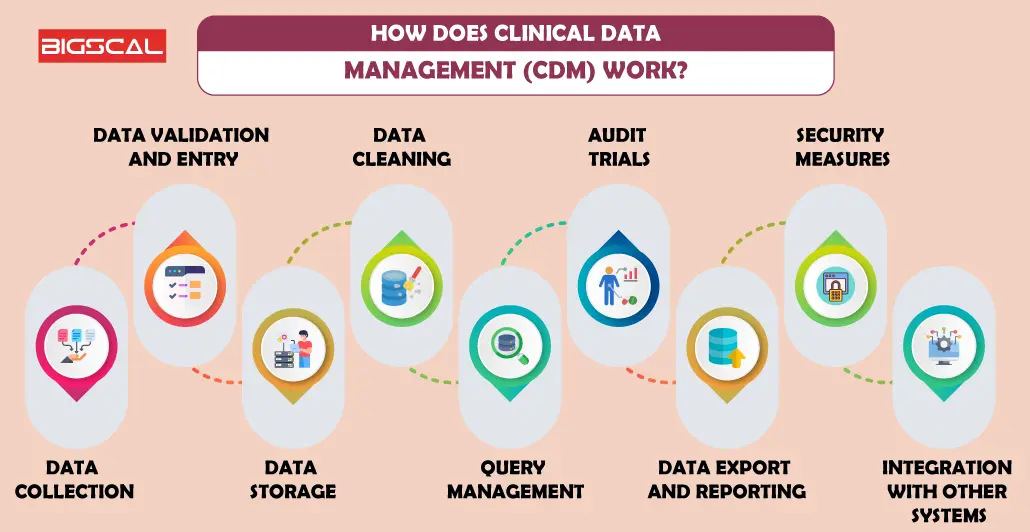
Data Collection
The CDMS also help clinicians in pulling out patient details during clinical trials systematically This enhances the ease of collection as the forms are readily filled on a search system. Such a practice entails attaining precision as well as uniformity absorbing the mistakes that would be likely associated with manual documenting. The data acquisition process is usually automated when the software integrates with various devices and systems to create optimal data communications that feed into clinical research, resulting in real-time trail updates and delays minimization in the study.
Data Validation and Entry
After data collection, accuracy and reliability of the information is a crucial tools for CDMs (computerized data management system). The software should point any incorrect data categorization via the data entry validation process which is specifically built inside it to assess the reliable data. This function of the data logger facilitates the retention of data accuracy by suggesting and highlighting the data errors through reviews and corrections. Moreover, as all the records are standardized in their information input, the occurrence of inaccuracies is significantly decreased.
Data Storage
CDMS files are able to keep enormous clinical data from the experiments. It has safely kept patient contact information, medical history and trial results in its secure databases. In these databases, the data is being structured and secured, hence the possibility of easy data retrieval and analysis exist for researchers. It is used with the help of the standardized format for the electronic data entry and capture. That way, data consistency are ensured regarding formats of data across various sites and studies.
Data Cleaning
Demedicalization (CDMS), for short, stands for the term that defined the process of locating and fixing the mistakes or inconsistencies that come up in clinical data. The software uses both automated data validation checks and manual profile reviews to ensure the ironing out of data anomalies such as a data mine, missing entries, high data inaccuracies. CDMS perform systematic cleaning of data which in turns boost the accuracy, non-ambiguity, and reliability of the data. The implementation of this procedure increases the accuracy and reliability of the study outcomes, minimizes the chances for errors that otherwise could occur and maintains compliance with regulatory requirements.
Query Management
The Query Management in CDMS offers, just general information, which refers to the data that are correctly uploaded or don’t have some bit of missing information. Finally, if uncertainty brings the situation to the statements that pin just the spots where unificaiton or reconstructing is needed, the decision is made. The significance of iterative procedures throughout this process is guaranteed by the fact that the final dataset is so precise and reliable, being reflective of the dependability of the data collected during the clinical trial.
Audit Trials
CDMS acts as dependent upon the purpose. Thus it can be an inspected and clinical trial database, and it has the characteristics of a detailed record of all activities within the system certified clinical data manager. This contains data processing, editing, and system touches from users. The utilization of records and audit trails is imperative for compliance purposes and quality assurance. They, being part of the watchdog mechanism, can easily track and verify all stages in the data management procedure. Audit Trails are serves as an underpinning of transparency, accountability, the trustworthiness of clinical trial data.
Data Export and Reporting
That is what a data extraction and representation tool is that is exporting data for further statistical analysis and reporting or to utilize tools for extracting valuable information during data transfer. The clinical studies researchers can develop data models reports and export the datas in all the formats for analysis purposes. The function of this generated synergy in turn helps to create collaboration, decision-making, and the communication of findings, which ultimately leads to the betterment and efficiency of clinical research.
Security Measures
CDM stresses security offenses so that patient information might be properly kept confidential. For instance, it means encryption methods, access controls, clinical data management systems and audit trails. Encryption is a security protection that covers data during its transmission and storage phases, and therefore it is not easily accessed by unauthorized persons. Access controls involve system admission to authorized personnel only and this ensures that only those with proper credentials i.e. documents, password, etc., are allowed to modify and manage data through system. Audit trails display user operations and this file contains a complete record of every user interaction system. Periodic security assessments and the subsequent vulnerability updates will additionally ensure that the software remains resistant to new and emerging risks.
Integration with Other Systems
Clinical Data Management Software integrates its data managers seamlessly with other systems. This integration enhances efficiency by enabling data exchange between CDMs and electronic health records (EHRs), laboratory information management systems (LIMS), clinic and electronic systems, clinic inventory management systems, and other relevant platforms.
Preferable Points Of Selecting CDMS
While selecting a clinical database management system, consider the following points:
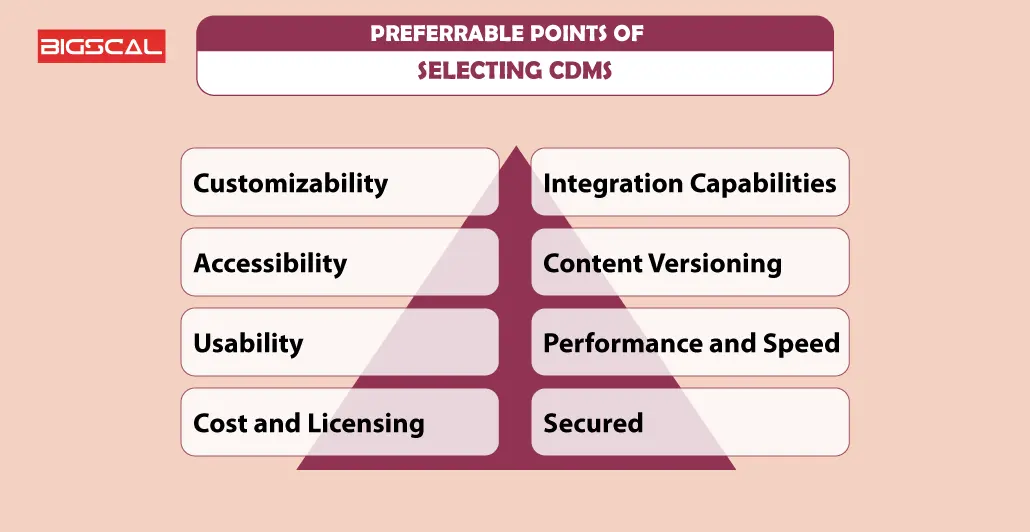
- Customizability
- Accessibility
- Usability
- Cost and Licensing
- Integration Capabilities
- Content Versioning
- Performance and Speed
- Secured
- Content Analytics
List Of Top Clinical Data Management Software

MainEDC™
MainEDC™ is one of the fact that Clinical Data Management Software use Leading and mainly to combines collecting and management of trial data. The system involves the promotion of data credibility, which corresponds to regulatory norms. As a result, we ensure that the provided trial results are valid and reliable. Unlike the past where there had to be extra time to deliver information or transfer data from one research team to another, the MainEDC™ platform deploys real-time collaboration among teams so that communication and data sharing become seamless.
Specialty and Benefits Of MainEDC™ Are:
- eCRF is a digital protocol for case reports.
- Data validation
- Drag-and-drop study designer
- The alignment of our data with other trial systems.
- Compliance Audit and Inspections I am responsible for.
- Two-way information access via MainEDC’s™ ePRO, whether mobile or web-based, for the patient.
- AI-powered medical coding
encapsia
Hence, encapsia is a clinical data management system that comes upfront with the latest innovation to make data handling in clinical trials easier and faster. It is a unison of the data collection, management and analysis processes which helps to unleash the complex aspects of clinical research. encapsia provides several functionality options such as customizable eCRFs, instant data visualization and statistics, auto-cleaning methods and more. Thus, this software is more profitable to the organization since it minimizes human errors and it increases the speed of data review.
Specialty and Benefits Of encapsia are:
- eSource
- Home visit
- Electronic Data Capture (EDC)
- Real-time updates
- Live and interactive data
- discrepancy management
- Insights
- Third-party data upload, review, and reconciliation
- Medical coding
- API integration
Dot Compliance
This CDMS works as a clinical data management workflow. It increases efficiency and optimizes data processes in the clinical research domain. Additionally, Dot Compliance facilitates efficient data collection, storage, and analysis, ensuring compliance with regulatory standards. With Dot Compliance, contract research organizations can confidently manage their clinical data and comply with industry regulations while establishing a more efficient and organized approach to data management.
Specialty and Benefits Of Dot Compliance are:
- Quality event management
- Training management
- Audit management
- CAPA management
- Risk management
- Complaint management
- Deviations/Non-conformances
- Supplier quality management
- Regulatory Information Management
- Electronic batch records
EDGE
EDGE is another most popular software that acts as a clinical data manager. It provides a secure and centralized platform for data collection. This allows clinical data management associations to capture and manage clinical trials efficiently. Additionally, EDGE supports real-time data entry and validation which minimizes errors and improves the data quality and accuracy. This software adapts to diverse workflows, promoting flexibility in study designs.
Specialty and Benefits Of EDGE are:
- Role based-access control and data encryption
- Real-time workflow process capture
- In-depth reporting capabilities
- Customizable fields
- Shared calendars
- Case report forms
- Statistically sound data
- Electronic responsibility delegation log
- Finance recording, tracking, and reports
VISION
VISION also helps in collecting, managing, and analyzing clinical trial data. It offers user-friendly data entry and validation interfaces, ensuring accuracy and compliance with regulatory standards. VISION enables efficient data integration, allowing researchers to collaborate and share insights easily. It is an advanced reporting tool that allows users to derive meaningful conclusions from complex datasets. Its secure and scalable architecture makes it suitable for various clinical research scenarios, enhancing overall research efficiency.
Specialty and Benefits Of VISION are:
- Form libraries
- DIY EDC builder
- Cross-form operations
- Create site forms
- Quick database lock
- Auto data cleaning
- On-demand reporting
- Archive in minutes
- Media upload
- Audit trail enabled
Castor EDC
Castor EDC, or Electronic Data Capture, is a versatile clinical data management software that simplifies data collection for research studies. It provides a customizable platform for creating electronic case report forms (eCRFs) without the need for coding skills. Castor EDC ensures data integrity through built-in data validation plans and checks, reducing errors in data entry. The platform supports seamless collaboration among research teams, allowing real-time data access and monitoring.
Specialty and Benefits Of Castor EDC are:
- Advanced eCRFs builder
- Database lock
- Flexible user management
- Audit trail
- Up to 2GB large file uploads
- Two-factor authentication
- Field-level encryption
- Automated pseudonym generation
- Disaster recovery report
- 15-year data retention
- ePRO surveys
RealTime CTMS
RealTime CTMS is a Clinical Data Management software that streamlines clinical trial planning, tracking, and other data management activities. It provides a user-friendly interface for researchers, helping them efficiently organize and monitor various aspects of their studies. This software enables easy patient data tracking, study progress, and regulatory compliance. RealTime CTMS facilitates communication among team members, ensuring everyone is on the same page regarding study milestones and tasks.
Specialty and Benefits Of RealTime CTMS are:
- Website and Facebook ads integration
- Mobile app referral options
- Financial tracking
- Configurable progressive recruitment statuses
- Patient referral metrics
- Automated reminders
- Email blast and mass texting campaigns
- Individual, departmental, and company calendars
- Task management
- Patient visit tracking
Florence eBinders
Florence eBinders is a user-friendly electronic binder system that simplifies the management of regulatory documents in clinical trials. It replaces traditional paper-based systems with a digital platform, making it easier for research teams to create, organize, and access essential documents. Florence eBinders promotes collaboration by allowing authorized personnel to securely share and review records, fostering a more efficient and compliant document workflow.
Specialty and Benefits Of Florence eBinders are:
- accelerates the document review process
- Online document management
- Remote access for sites, monitors, and auditors
- Full compliant eSignatures
- Permission-based access control
- Project management
- Flexible and custom binder structures
- CTMS integration
- Outlook email integration
- Automated filing of centralized documents into eBindersn
- EMR integration
Clinical Conductor
It streamlines and organizes clinical trial processes. In addition, it offers a user-friendly interface for researchers, allowing them to manage study data, participant information, and regulatory compliance efficiently. The software facilitates seamless collaboration among research teams, ensuring accurate and secure data collection. It also aids in maintaining regulatory compliance by using standard operating procedures and providing tools for audit trails and documentation. The software’s intuitive design minimizes the learning curve for users, making it accessible for both experienced researchers and those new to clinical data management.
Specialty and Benefits Of Clinical Conductor are:
- Electronic consent
- eRegulatory Management System
- eSource
- EDC
- Research ROI reporting
- Automated participant payments
- Dedicated platform for sponsors, sites, and participants
- Document sharing
ClinCapture
ClinCapture is a leading clinical data management software known for its flexibility and scalability. This platform enables researchers to efficiently design and manage electronic case report forms (eCRFs). ClinCapture’s user-friendly interface empowers researchers to create customized data collection forms tailored to their specific study requirements. The software supports a wide range of data types, including text, numeric, and date fields, ensuring versatility in data collection.
Specialty and Benefits Of ClinCapture are:
- ePRO
- eCOA
- eConsent
- Randomization and trial supply management
- eSource
- Medical coding
- Form versioning
- Risk-based monitoring
- Advanced integrations
- Standard and advanced reporting and analytics
How Bigscal Can Help In Developing Clinical Data Management Software?
If the above CDMS won’t work for you, develop your own data management solution with us. We can assist you in developing emerging clinical data management software by providing scalable solutions. We have expertise in developing solutions that can organize, store, and manage large volumes of clinical data efficiently. Our software also simplifies the process of collecting, analyzing, and cleaning data from clinical trials. It will ensure accuracy and compliance with regulatory standards.
So, without further delay, reach out to us now.
Conclusion
Clinical data management requires embracing software systems that integrate the latest features and follow trends. All the above CDMS/CTMS are best and will be demanding in upcoming years. Their features, user-friendly interface, and commitment to data security make it a promising choice for streamlining clinical data processes.
FAQ
Clinical data management solutions?
High-level solutions on clinical data management CDM leads to Medidata, IBM clinical development and Oracle clinical. They have an array of capabilities that control sharing of data, implement robust security to protect data and enforce industry standards. The friendly user interface and the sophisticated analytics of these new technologies go a long way to ease the operations of clinical trials, improve corresponding efficiency, and provide solid assurance of essential data use in medicine.
What is clinical data management?
Clinical Data Monitoring implies collecting, storing, and integration of clinical trial and health survey data. This process forms the core of the data gathering strategy, which is an integral part of the generation of accurate and quality data, which will be beneficial in the undertaking of research and ultimately, the formulation of informed decision-making in the healthcare sector.
What are the 4 phases of clinical data management?
The basic principles of clinical data management are the stipulation of data acquisition, processing, checking, and analysis. During data acquisition locating is placed in clinical trials. Data entry constitutes of inputs into the databases of these data. Data cleansing guarantees exactness, and data mining provides deep insights on the cleaned and processed data.
What skills do you need to be a clinical data manager?
The data information manager should perform data analytics, pay attention to minute details, be learned about international regulations pertaining to the information and be fairly proficient in data management software. The attributes like good talking, problem solving and organizing are a must. The world underrider refers to having interdisciplinary teams which help them to play a role of world underrider.
What is data management in a clinical trial?
To the data aboard, in the clinical trial it is taking place systematically collecting, organization and verification of information generated during the experiment is referred to. It gives us the ability to verify the accuracy of data, completeness of information, as well as ability to observe in compliance with regulatory standards. This procedure is full of importance for preserving the data-integrity of such trials and for data-analysis aimed at conclusions-drawing or inference-making.







